Medical Device Registration
We have experts of regulatory and registration affairs who have more than 20 years of experience in product registration and are familiar with the local environmental and technical requirements of investigational product registration in China, and will provide our clients with product registration service of medical devices and diagnostic reagents. While providing feasible product registration strategy and pathway, we also build a good communication bridge between enterprises and drug regulatory authorities to ensure efficient communication between the two sides. Our service scope includes:
- Consultation about laws and regulations on registration;
- Feasibility evaluation and guidance for registration of investigational product in China;
- Review and instruction for improvement of application dossiers;
- Sorting out (including translation) and submission of application dossiers;
- Quality standard recheck;
- Whole-process tracing and coordination for communication during registration;
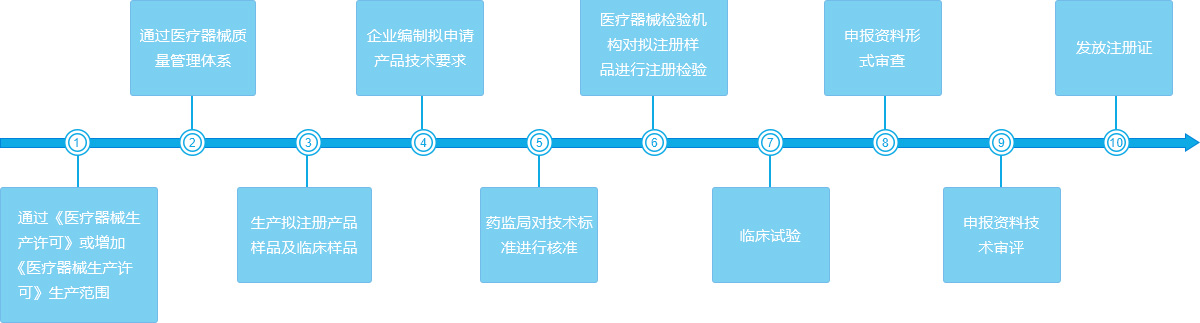
Medical device first registration flow chart