Our team have professional background and rich experience in drug registration and are familiar with the regulations issued by Chinese regulatory authorities, have an accurate understanding of industry trends, and provide our partners with the most valuable registration strategy and service by communicating with experts in the industry and administration authorities.
The registration team understands and is familiar with various approval processes, covering areas such as pharmaceuticals, medical devices, and health foods. And communicate with the competent authorities at all levels to help your products go on the market in China. The services of the Regulatory and Registration Team include:
- Consulting service: drug registration feasibility survey
- Registration service: application for registration of drugs (specifically, registration service includes: dossier review, dossier translation and proofreading, dossier compilation and writing, application submission, quality standard recheck, whole-process tracing of review progress, and problems solving during the review etc.).
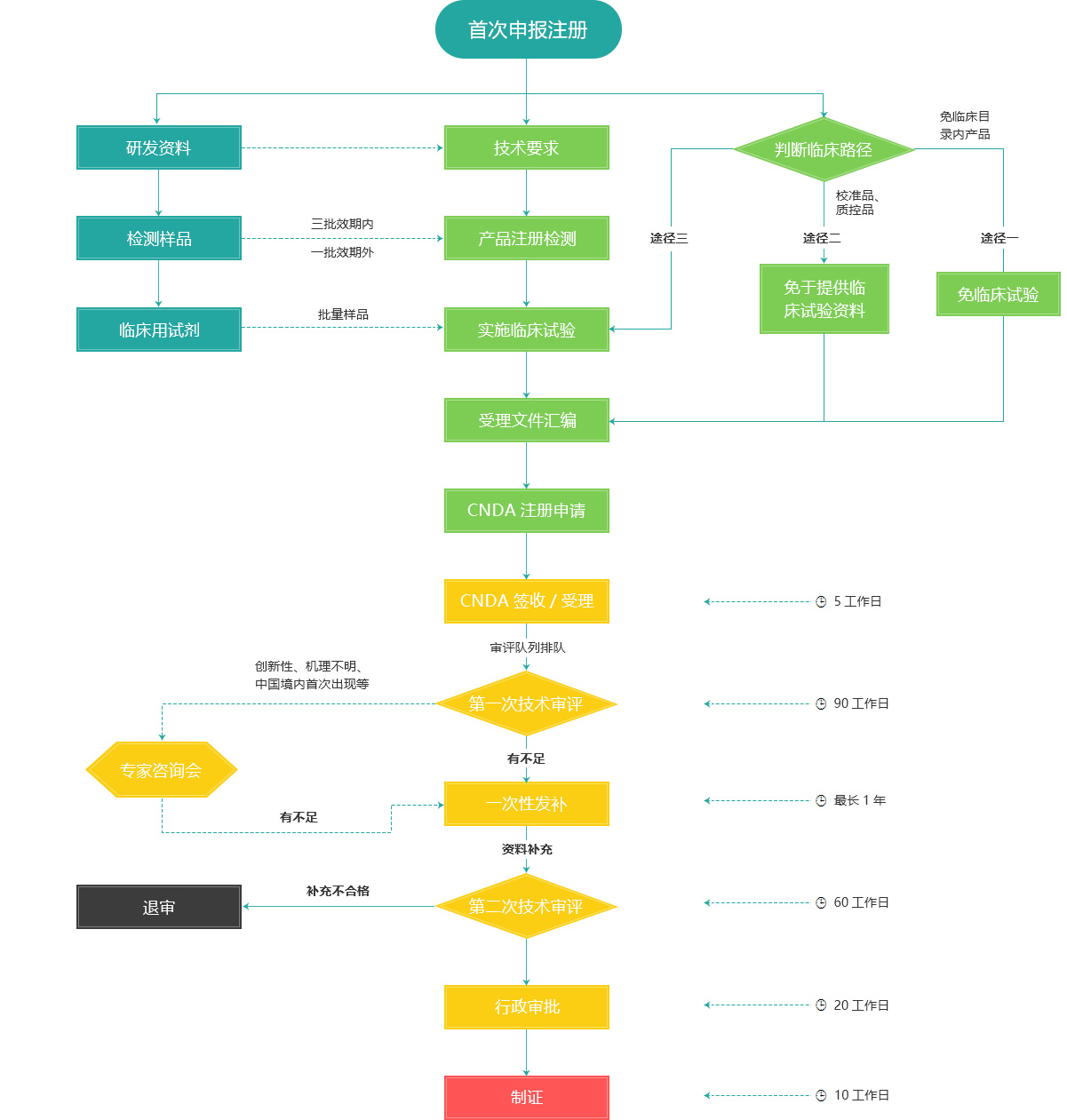
China's Class III IVD product first registration process